Abstract
Background: Treatment advances over the past two decades have improved overall survival in patients with multiple myeloma (MM). However, early mortality continues to be an issue that requires better characterization. We sought to explore the impact of newer therapeutics and practices on early mortality (within 2 years) with the latest SEER dataset. Using the dataset, we also evaluated whether improvement in early mortality differed based on race-ethnicity.
Methods: We identified 78,121 patients with MM from the SEER Research Plus Data 17 Registries (2000-2019) in SEER*Stat 8.4.0. Cases were selected by the following criteria: histologic type (ICD-O3 = 9732-9733), microscopically confirmed, diagnosis in 2000-2017, and age from 18-99 years old. Logistic regression was performed to assess relationship with early mortality, defined as overall survival less than or equal to two years (Table 1). Age, gender, self-identified race-ethnicity, cause of death (COD), socioeconomic status (SES), year of diagnosis, and geographic region of diagnoses were all variables initially evaluated. The final logistic regression model was then based on age, gender, self-identified race-ethnicity, and year of diagnosis (split into three-year groups) as predictors. The first quartile age group (18-58), male gender, non-Hispanic White (NHW) race, and year of diagnosis between 2000-2002 were utilized as reference groups. Analysis was performed in RStudio 4.1.2.
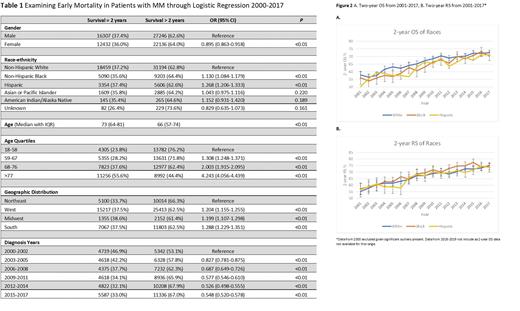
Of the predictors chosen in the logistic regression model, race-ethnicity was examined further. Yearly 2-year overall survival (OS) and relative survival (RS) of MM from 2001-2017 by race-ethnicity with 95% confidence intervals were exported from SEER*Stat 8.4.0 (Figure 2). We also specifically looked at 2-year RS to evaluate for cancer-specific survival in absence of other causes of death.
Results: Female patients with MM had lower odds of experiencing early mortality (OR 0.895, 0.863-0.918, p=<0.01). Non-Hispanic Blacks (NHB) and Hispanics had higher odds of experiencing early mortality (OR 1.130, 1.084-1.179, p=<0.01 and OR 1.268, 1.206-1.333, p=<0.01). Older age was also associated with increased early mortality. The odds of early mortality appeared greater in regions outside the northeast. Finally, the odds of early mortality decreased for patients diagnosed with MM between 2000-2014, but stayed stable in 2015-2017. Examining 2-yr OS and RS from 2001-2017, there is overall improvement during the period as well, but the differences between race-ethnicities (NHW vs NHB and NHW vs Hispanics) were not statistically significant.
Conclusion: The introduction of novel therapies has revolutionized the care of patients with MM. This is reflected by our data showing overall decreasing odds of early mortality from 2000-2014. However, there is no significant improvement further in 2015-2017. Importantly, we see improvement in 2-year survival (cancer-specific survival and OS) for all racial-ethnic groups over time. This trend overall parallels the FDA approvals of novel therapies such as bortezomib in 2003, lenalidomide (Revlimid) in 2006, and daratumumab in 2015.
In addition, we note some disparities in terms of early mortality based on race-ethnicity, age, and region. Further investigation is warranted to explore the etiologies behind these disparities, possibly evaluating disease biology, treatments, comorbidities, and insurance information.
Disclosures
Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah:MashUpMD: Honoraria; MJH Lifesciences: Consultancy, Honoraria; Janssen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; ACCC: Honoraria; Sanofi: Consultancy. Janakiram:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Verma:Throws Exception: Current holder of stock options in a privately-held company; Jannsen: Consultancy, Research Funding; Eli Lilly: Research Funding; Medpacto: Research Funding; Ionctura: Research Funding; Stelexis Therapeutics: Current equity holder in private company, Honoraria; Bakx Therapeutics: Consultancy, Current equity holder in private company; Prelude: Research Funding; Novartis: Consultancy; BMS: Research Funding; Curis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal